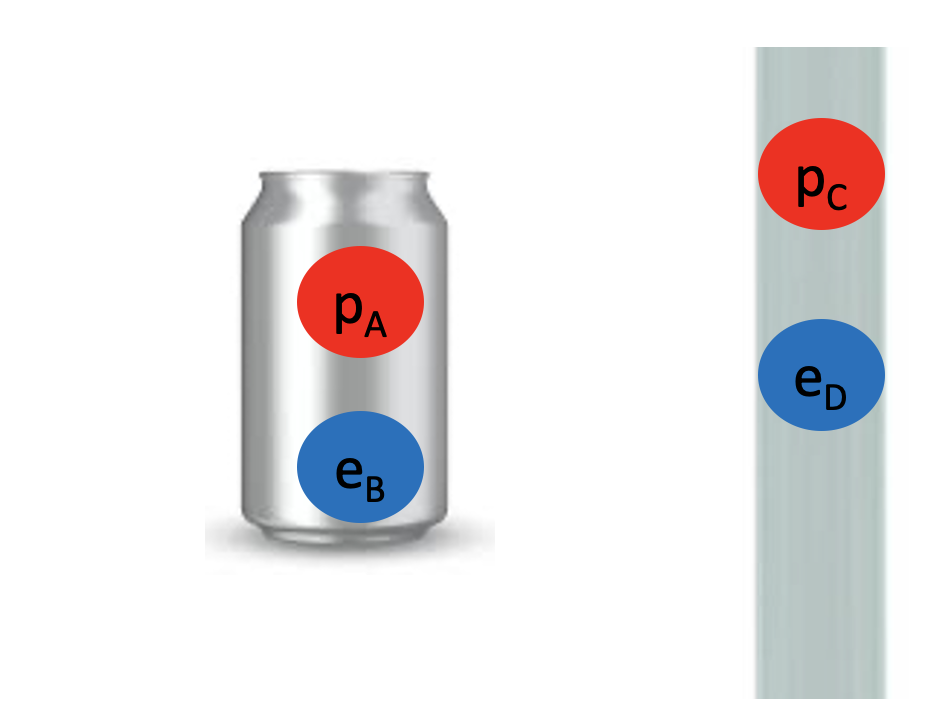
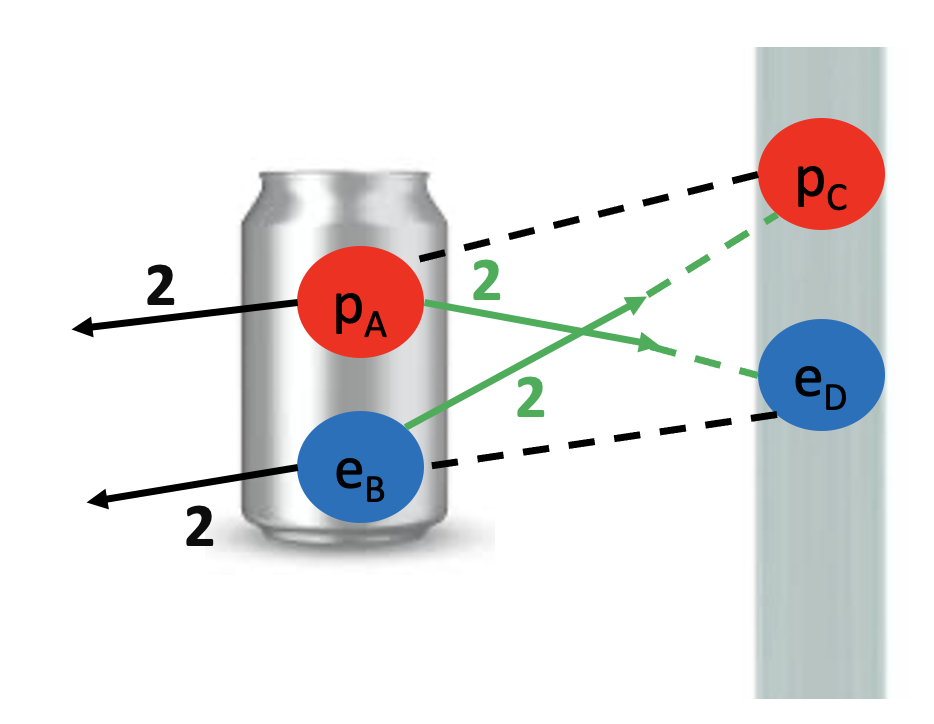
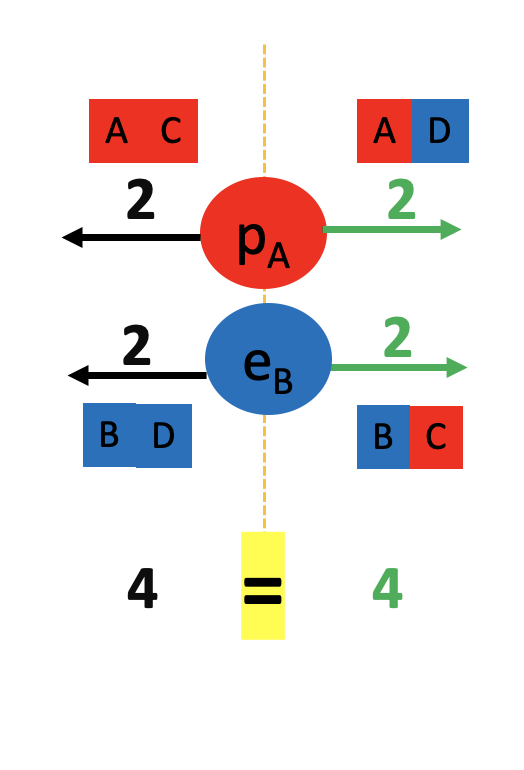
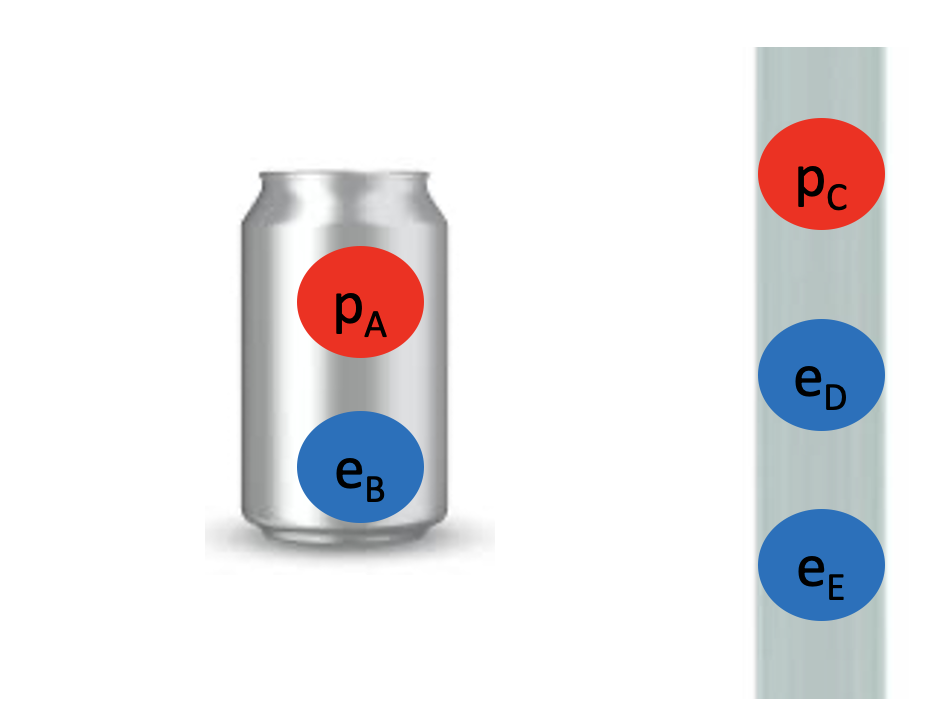
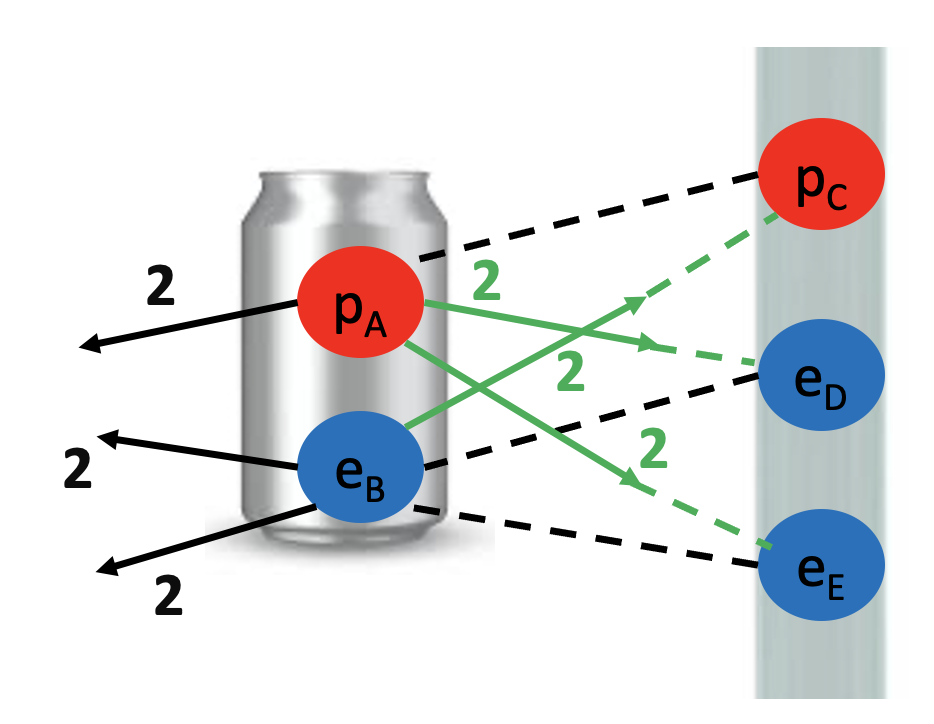
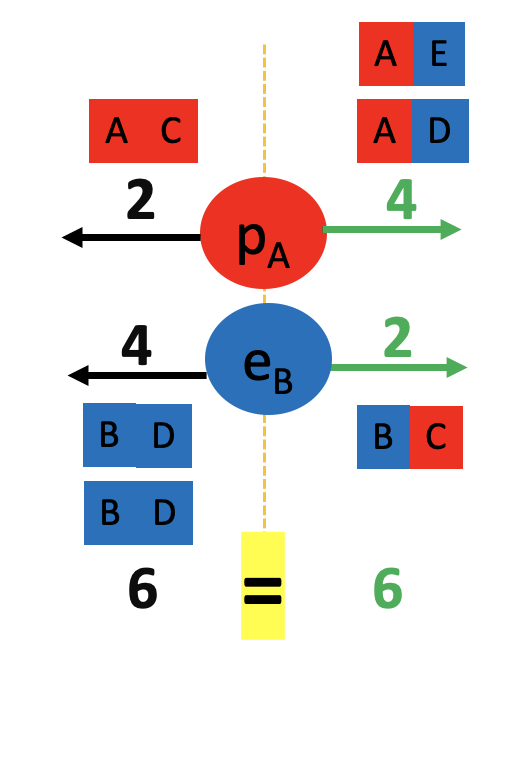
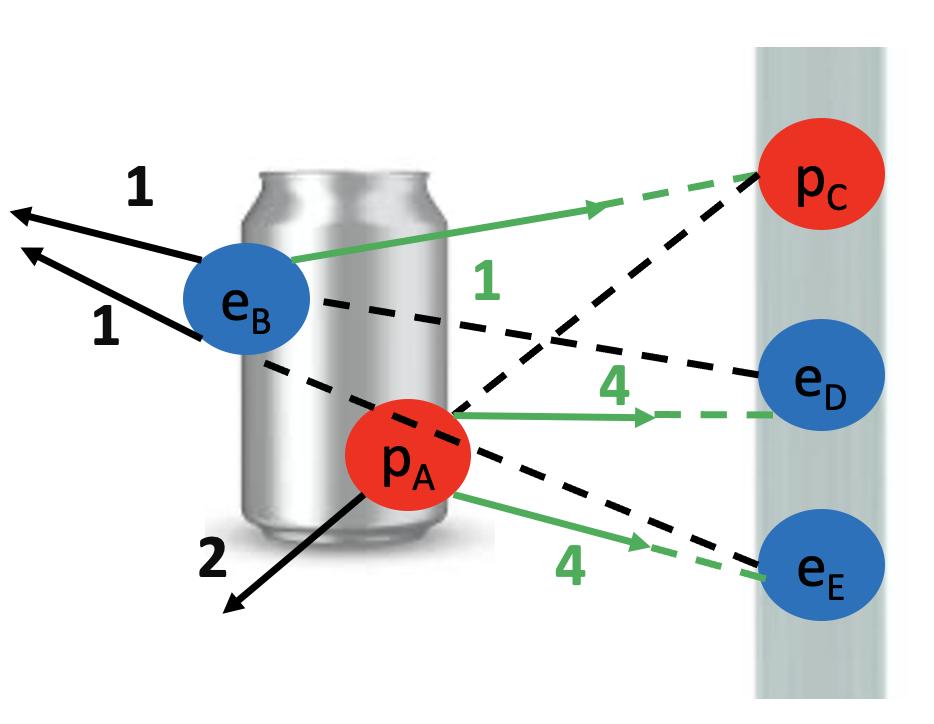
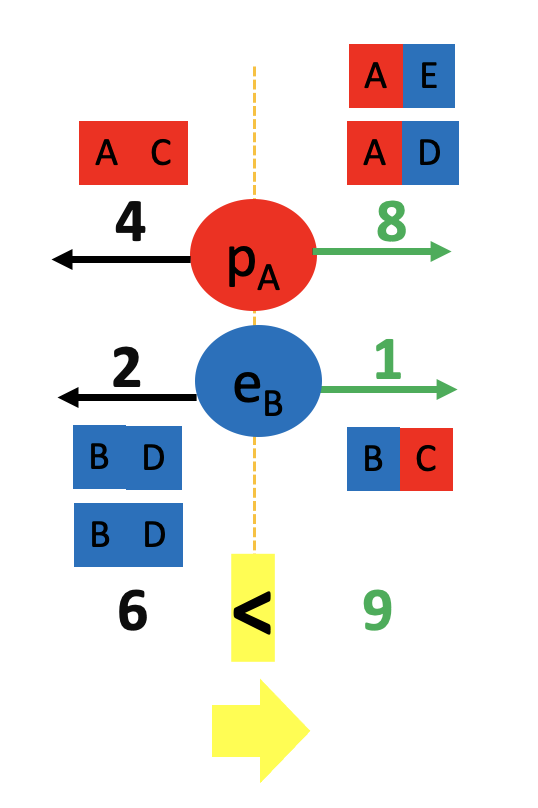
We started by observing a pipe that attracts a can and bends water!
What do these two examples have in common? The pipe, the can and the water all have electrons and protons! Rubbing the pipe with the cloth charges the pipe with electrons. They attract the protons in the can and in the water. The can rolls off the table towards the pipe and the water bends towards the pipe too! Let’s see the math in more detail: