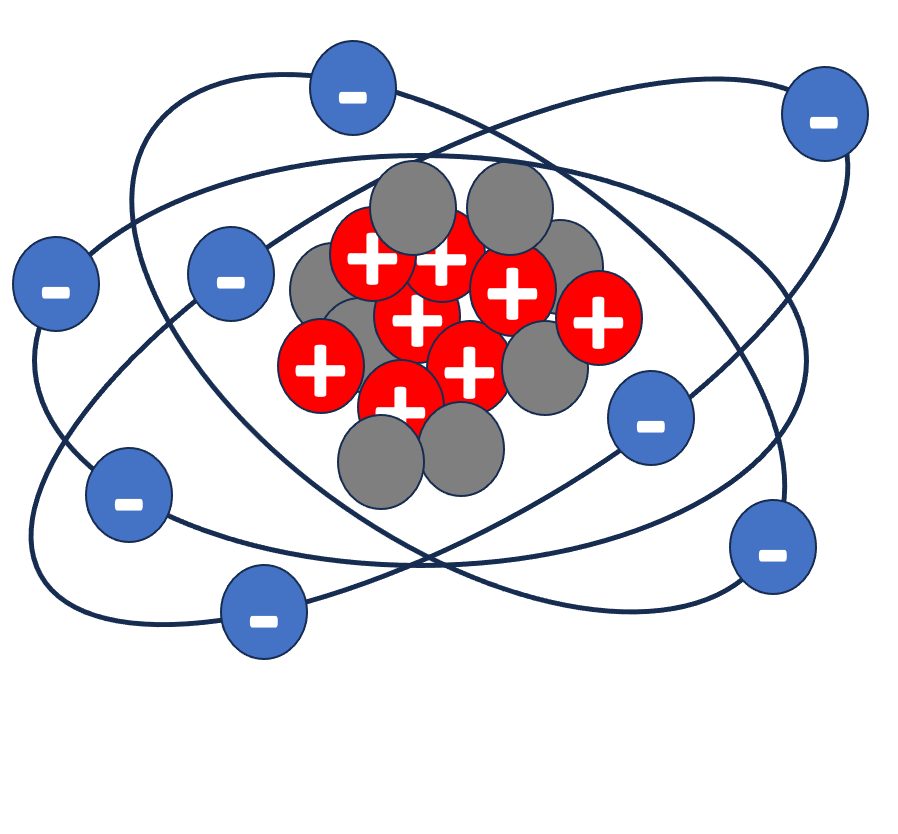
Matter is made out of atoms. Atoms have neutrons, and protons in the nucleus, and electrons that orbit around it. Neutrons have no electric charge. Protons are charged positively and electrons are charged negatively. Protons and neutrons have mass. Electrons have almost no mass. Here is an example:
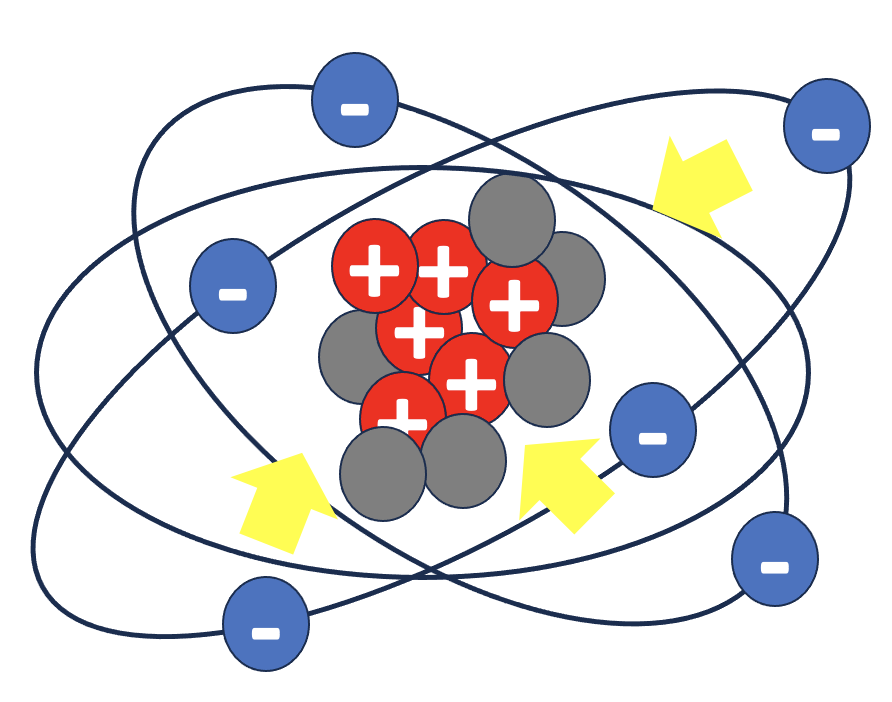
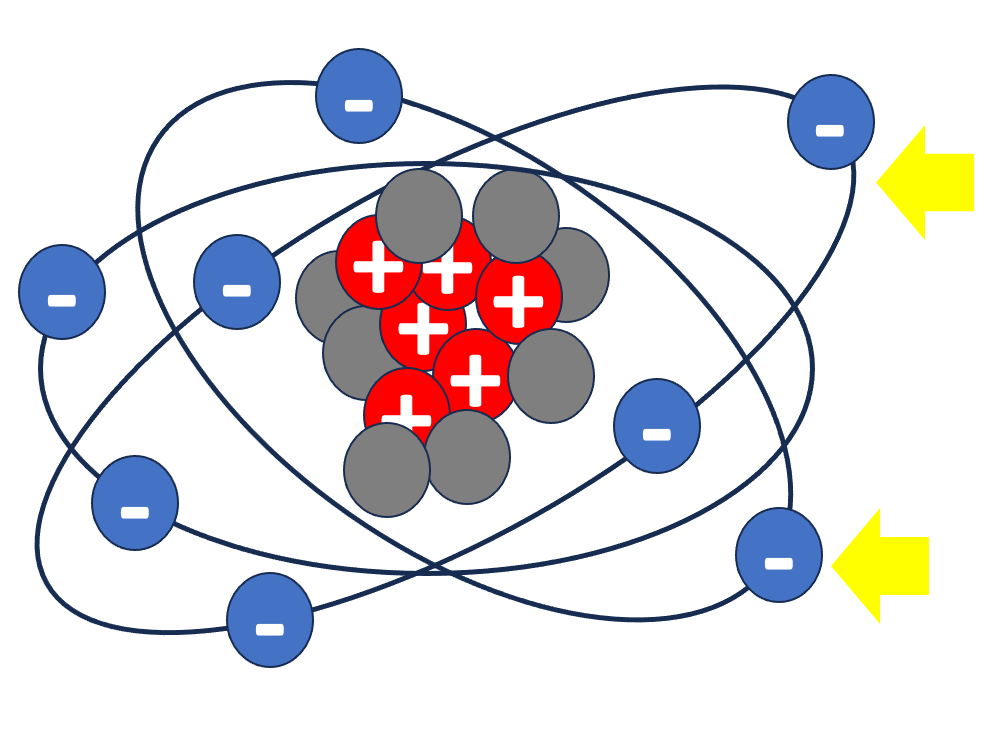
Charges of similar valence repel each other while charges with opposite valence attract each other. See the examples below. The force is bigger if there are more protons and more electrons or if they are closer to each other. In an atom, the protons attract the electrons keeping them tight orbiting the nucleus (represented above by the arrows).



Elements are defined by their atomic number, which is the number of protons in the nucleus. Here are some examples. The atom of Hydrogen has 1 proton, 1 electron, and no neutrons. The atom of Oxygen has 8 protons, 8 electrons, and 8 neutrons. The atom of aluminum has 13 protons, 14 neutrons, and 13 electrons.
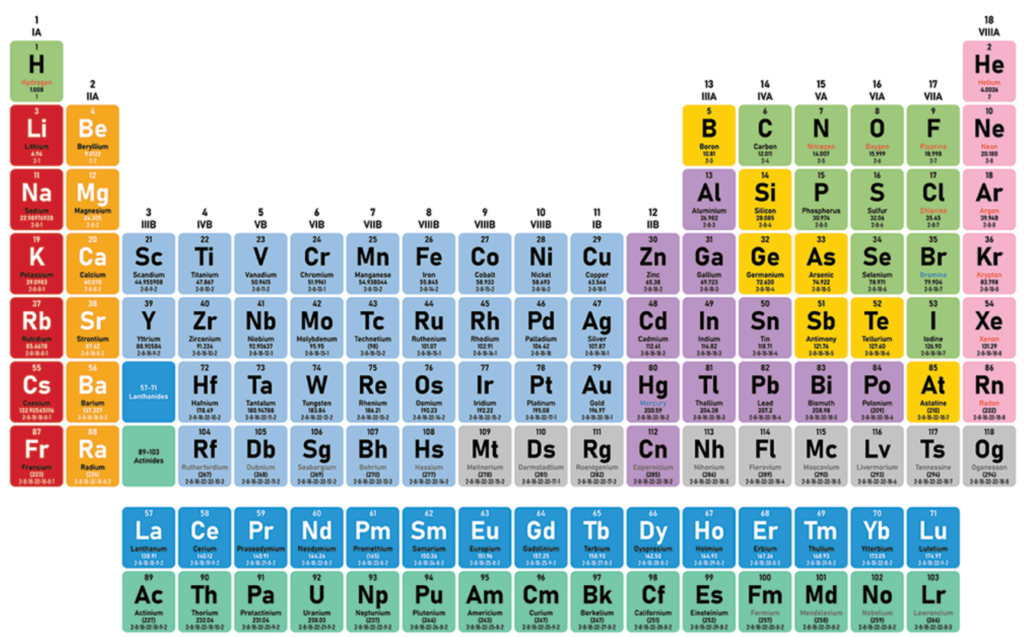
Elements have names, symbols, atomic number and weight. They are all organized into the periodic table, which provides all this information. The elements in the same row have similar space in their orbits to receive electrons from other atoms, or similarly crowded orbits and then a tendency to lose electrons to other atoms. Insulators are more like to receive electrons, while conductors are more likely to lose electrons.
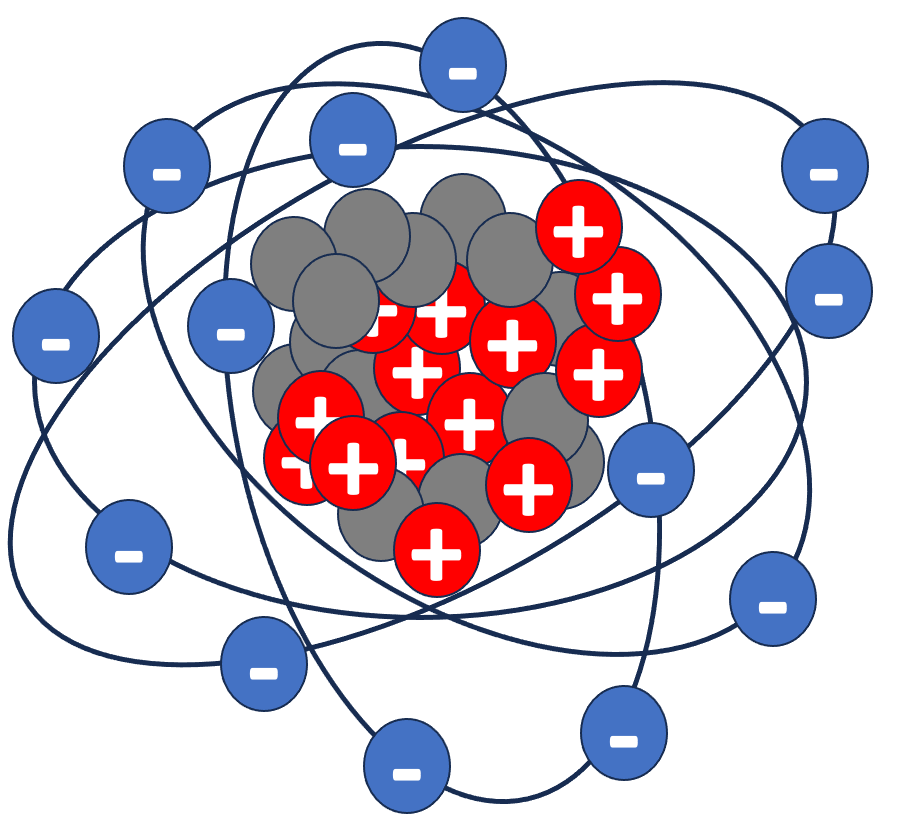
Sometimes electrons are free to roam across atoms. An atom can be negatively charged, if there are more electrons than protons, called cation, or positively charged, if there are more protons than neutrons, called anion. Here are some examples: